


TrialKit - eClinical Platform

TrialKit - eClinical Platform의 설명
Designed for both Study Participants and Clinicians alike - Leverage the latest in EDC/CTMS technology to expedite clinical trials and advance healthcare. TrialKit eliminates the need for costly, outdated data collection tools by embracing the accessibility and efficiency of today's mobile world.
The mobility and versatility of TrialKit allow for seamless regulatory compliant (21 CFR Part 11) data capture from mobile devices anytime, anywhere. Once collected, data can be easily aggregated, analyzed and shared, making collaboration amongst research teams more productive.
Best of all, no programming expertise is needed to use TrialKit, so any research professional can create a study using an iOS device.
How does it work?
1. Build a study
An intuitive user interface enables study builders to create electronic case report forms (eCRFs) to collect essential study data in accordance with the study protocol and regulatory requirements. Ensure accurate data collection with easy-to-create edit checks.
2. Collect data
TrialKit enables research teams to collect and cleanse clinical data from virtually any location. Export data in a variety of formats for review and submission and capture ePRO data with ease.
3. Manage workflow
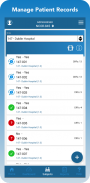
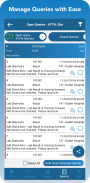
With TrialKit, clinical research can be expertly managed from start to finish on an iPhone or iPad. Configuring role-based security, form review levels, and risk-based monitoring is simple. The ability to access, monitor, and review data or respond to queries on-the-go is now a reality.
4. Engage study participants
The ePRO capabilities built into TrialKit will enhance your clinical trials. Automate the entire notification process and enforce patient survey completion by sending reminders. Study participants’ access has been made simple and flexible, so little or no training is required. Email and/or in-app notifications guide patients directly to their surveys, which can be accessed through the app or through any web browser.
5. Analyze data
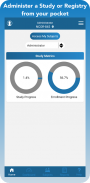
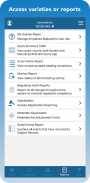
Robust reporting options enhance the ways research teams view and analyze data gathered in clinical trials. In addition to My Queries, Action Items, Regulatory Audit, and Outcome Summary reports, TrialKit generates a dynamic, visual report to track study progress in real-time.
Unique Features and Functionality:
Device-targeted eCRF design
Image and video capture directly to eCRFs
Site and study document support
Built-in ePRO, including a patient handoff mode
Adjudication for endpoints, outcomes, and inclusion/exclusion criteria
Inventory management - Manage both drug and device inventory with a device you carry in your pocket. Barcode scanning eliminates human error and gives you real-time drug and or device disposition.
To learn more about reducing the cost of clinical research using TrialKit, visit www.trialkit.com.





















